A breast implant is a prosthesis used to enlarge the size of a woman's breasts (known as breast augmentation, breast enlargement, mammoplasty enlargement, augmentation mammoplasty or the common slang term boob job) for cosmetic reasons.
An implant can also be used to reconstruct the breast (e.g. after a mastectomy; or to correct genetic deformities), or as an aspect of male-to-female sex reassignment surgery.
According to the American Society of Plastic Surgeons, breast augmentation is the most commonly performed cosmetic surgical procedure in the United States. In 2006, 329,000 breast augmentation procedures were performed in the U.S.
There are two primary types of breast implants: saline-filled and silicone-gel-filled implants. Saline implants have a silicone elastomer shell filled with sterile saline liquid. Silicone gel implants have a silicone shell filled with a viscous gel. There have been several alternative types of breast implants developed, such as polypropylene string or soy oil, but these are uncommon and not recommended.Breast augmentation, plastic surgery dublin, cosmetic surgery dublin, plastic surgery, cosmetic surgery
 PROCEDURE
PROCEDURE
The surgical procedure for breast augmentation takes approximately one to two hours. Variations in the procedure include the incision type, implant material, and implant pocket placement.
INCISION TYPES
Breast implants for augmentation may be placed via various types of incisions:
Inframammary - an incision is placed below the breast in the infra-mammary fold (IMF). This incision is the most common approach and affords maximum access for precise dissection and placement of an implant. It is often the preferred technique for silicone gel implants due to the longer incisions required. This method can leave slightly more visible scars in smaller breasts which don't drape over the IMF.
Periareolar - an incision is placed along the areolar border.
Transaxillary - an incision is placed in the armpit and the dissection tunnels medially.
Transumbilical (TUBA) - a less common technique where an incision is placed in the navel and dissection tunnels superiorly. This approach enables implants to be placed with no visible scars on the breast, but makes appropriate dissection and implant placement more difficult.
Transumbilical procedures may be performed bluntly or with an endoscope (tiny lighted camera) to assist dissection. This technique is not appropriate for placing silicone gel implants due to potential damage of the implant shell during blunt insertion.
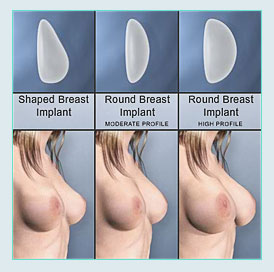 TYPES OF IMPLANTS
TYPES OF IMPLANTS
SALINE IMPLANTS
Saline-filled breast implants were first manufactured in France in 1964, introduced by Arion with the goal of being surgically placed via smaller incisions. Current saline devices are manufactured with thicker, room temperature vulcanized (RTV) shells. These shells are made of silicone elastomer and the implants are filled with salt water after the implant is placed in the body. Since the implants are empty when they are surgically inserted, the scar is smaller than is necessary for silicone gel breast implants (which are filled with silicone before the surgery is performed). A single manufacturer (Poly Implant Prosthesis, France) produced a model of pre-filled saline implants which has been reported to have high failure rates in vivo.
Saline-filled implants were most common implant used in the United States during the 1990s due to restrictions that existed on silicone implants, but were rarely used in other countries. Good to excellent results may be obtained, but as compared to silicone gel implants, saline implants are more likely to cause cosmetic problems such as rippling, wrinkling, and to be noticeable to the eye or the touch. Particularly for women with very little breast tissue, or for post-mastectomy breast reconstruction, silicone gel implants are considered as superior. In patients with more breast tissue in whom submuscular implant placement is used, saline implants can look very similar to silicone gel.
SILICONE GEL IMPLANTS
Thomas Cronin and Frank Gerow, two Houston plastic surgeons, developed the first silicone breast prosthesis with the Dow Corning Corporation in 1961. The first woman was implanted in 1962. Silicone implants are generally described in terms of five generations which segregate common characteristics of manufacturing techniques.
First generation
The Cronin-Gerow implants were made of a silicone rubber envelope (or sac), filled with a thick, viscous silicone gel with a Dacron patch on the posterior shell.[17] They were firm and had an anatomic "teardrop" shape.
Second generation
In response to surgeons' requests for softer and more lifelike implants, breast implants were redesigned in the 1970s with thinner, less cohesive gel and thinner shells. These implants had a greater tendency to rupture and leak, or "bleed" silicone through the implant shell, and complications such as capsular contracture were quite common. It was predominantly implants of this generation that were involved in the class action-lawsuits against Dow-Corning and other manufacturers in the early 1990s.
Another development in the 1970s was a polyurethane foam coating on the implant shell which was effective in diminishing capsular contracture by causing an inflammatory reaction that discouraged formation of fibrous tissue around the capsule. These implants were later briefly discontinued due to concern of potential carcinogenic breakdown products from the polyurethane.[18] A review of the risk for cancer from TDA by the FDA later concluded that the risk was so small so as not to justify recommending explantation of the devices from individual patients. Polyurethane implants are still used in Europe and South America, but no manufacturer has sought FDA approval for sale in the United States.[19] Second-generation implants also included various "double lumen" designs. These implants were essentially a silicone implant inside a saline implant. The double lumen was an attempt to provide the cosmetic benefits of gel in the inside lumen, while the outside lumen contained saline and its volume could be adjusted after placement. The failure rate of these implants is higher than for single lumen implants due to their more complex design. The contemporary versions of these devices ("Becker Implants") are used primarily for breast reconstruction.
Third & Fourth generation
Third & fourth generation implants, from the mid 1980s, represented sequential advances in manufacturing principles with elastomer-coated shells to decrease gel bleed, and are filled with thicker, more cohesive gel. These implants are sold under restricted conditions in the U.S. and Canada, and are widely used in other countries. The increased cohesion of the gel filler reduces potential leakage of the gel compared to earlier devices. A variety of both round and tapered anatomic shapes are available. Anatomic shaped implants are uniformly textured to reduce rotation, while round devices are available in smooth or textured surfaces.
Fifth generation
Evaluation of "gummy bear" or solid, high-cohesive, form-stable implants is in preliminary stages in the United States but these implants have been widely used since the mid 1990s in other countries. The semi-solid gel in these type of implants largely eliminates the possibility of silicone migration. Studies of these devices have shown significant potential improvements in safety and efficacy over the older implants with low rates of capsular contracture and rupture.
>> Should you have any further questions, please contact us






